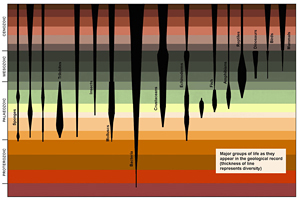
At the beginning of the Cambrian Period, within a span of a mere 10 million years, all the major groups of complex animal life, all the phyla, appeared. Ten million years may seem like a vast stretch of time: by most criteria it is a lot of time. But consider that nearly 3 billion years had already gone by since life had left its first traces in the fossil record. And consider, too, that no new phyla are known to have originated since the early Cambrian.
Here again we find a familiar pattern, on a truly grand scale: relatively suddenly, the whole spectrum of invertebrate life, including sponges, brachiopods, arthropods (trilobites, chelicerates and crustaceans), mollusks, plus spineless chordates in the same phylum as the vertebrates, burst on the scene, the world over. By the end of the Cambrian we have records for all the major groups of hard-shelled invertebrate organisms, and some evidence that vertebrates had appeared as well.
What could have caused such a proliferation?
Niles Eldredge, Fossils: The Evolution and Extinction of Species, 1991 (p 189).
The creativity represented by this invasion of novelty was so extravagant that there were more phyla in the Cambrian, at the beginning of the animal record, than at any future time, despite the fact that the entire evolutionary history of animals was yet to come. One locality where the conditions for preservation were exceptionally favourable, Chengjiang in South China, records no fewer than 18 phyla. Only one new phylum emerged after the Cambrian; many went extinct – which only adds to the sense of strangeness.
Organisms are classified according to their similarities, the lowest unit of classification being the species. Species comprise individuals that are most similar to each other. Typically, a small morphological gap separates one species from the next closest species. Species that are the most similar to each other are then grouped into genera, genera into families, families into orders, orders into classes, and classes into phyla. Not all phyla are so diverse as to include families, orders and classes. A single species may constitute a phylum all by itself if no other species share its distinctive design features.
Such a scheme is reasonably understood to reflect degrees of genealogical relationship. Over time species multiply and diversify, with the result that recent species are more similar to each other than to older species. Gaps between the groups increase as one ascends the hierarchy, while the number of groups decreases: classes, for example, differ more than orders do, and phyla differ more than classes. That said, the greater the morphological and molecular differences, the more one needs to ensure that solid evidence supports the assumption of genealogical relationship. Otherwise the gaps may be evidence of non-relationship.
The theory of evolution assumes no limits. When phyla are grouped into kingdoms and kingdoms into domains (eukaryotes, bacteria and archaea), the assumption is that all organisms are related, no matter how different. Eukaryotes comprise all organisms whose cells contain a nucleus, namely animals, plants, fungi and protists: it is believed that animals are related to fungi simply because their cells all have a nucleus. Archaea, ubiquitous single-celled microbes whose architectural distinctiveness was recognised only in 1977, differ radically from both bacteria and eukaryotes. Among their differences is the way they package their DNA into flexible coils, enabling the information to be accessed without the need for special proteins. The ultimate step in this reductive chain of reasoning is to postulate that these three domains arose from an entity called the ‘last universal common ancestor’, or LUCA. Unfortunately, LUCAs, do not exist, and whether they ever existed is something evolutionary biologists themselves find hard to swallow. Archaea and bacteria are genetically so different that any such ancestor would have had to have more genes and metabolic capabilities than either of them, a kind of super-cell whose existence would only aggravate the problem of how the LUCA itself evolved into being. Some have suggested that the tree of life be replaced by a circle, a web, or a network (Katz et al. 2021). Nor should one suppose that archaea and bacteria are just evolutionary leftovers. They play a vital role in the biosphere, not least in the human gut. Also on the skin, where bacteria, fungi, viruses and archaea skin communicate with immune cells and with each other to keep the skin healthy and ward off pathogens.
If there is a limit beyond which genealogical relationship ceases to be plausible, where might that be? In most cases, the largest grouping suggestive of common ancestry is the phylum. Within a phylum, organisms are united by a body plan or architectural design that differs fundamentally from other body plans. When phyla are grouped with other phyla, the body plan dissolves and one is left with an uninformative generality. When phyla are grouped with other phyla, one is left having to plumb the fundamentals of how body plans are assembled: whether the embryo begins with two germ layers or three, whether the mouth or the anus forms first, whether the animal is radially or bilaterally symmetrical. The body plan dissolves and one is left with uninformative generality.
Invertebrates can be classified into around 35 basic body plans, ignoring extinct phyla and not counting phyla that have been lumped together (e.g. trilobites, onychophorans, anomalocarids, chelicerates, crustaceans and uniramians within the phylum Arthropoda), and they are distinct from the moment they appear. In the context of Darwinian evolution ‘basic body plans’ are not predicted. Darwinian evolution postulates that life starts with minimal complexity and becomes more complex gradually, whereas in fact, at both the morphological and molecular level, all animals are complex, even those at the beginning of their fossil record. This is because animals move, and locomotion requires a locomotor system (muscles, ligaments, tendons and the like), a nervous system capable of sensing the environment, energy produced by digestive and respiratory systems, an excretory system and (almost invariably) a brain. So far as we can tell from fossils, the phyla that have eyes now had eyes then, because as legged or swimming animals they needed them to navigate. Some eyes were extremely complex. The stalked eyes of one arthropod consisted of over 2000 lens facets (Zhao et al. 2013). Another arthropod fossil, also from the Early Cambrian, shows that some compound eyes had over 3000 lenses (Lee et al. 2011). In both cases, the size gradient of the lenses created a distinct ‘bright zone’, where the visual field was sampled with higher light sensitivity, as seen in modern taxa such as dragonflies. Consider how much brain power is needed to process 3000 images, continually changing as one moves about. The genetic programs that constructed all this complexity were, necessarily, no less complex; it is implausible to suppose they could have been the products of mutations haphazardly accumulating one by one.
The fossil record suggests that the major pulse of diversification of phyla occurs before that of classes, classes before that of orders, and orders before families. … The higher taxa do not seem to have diverged through an accumulation of lower taxa.
Evolution 41, 1177–1186 (1987)
When cnidarians first appear in any numbers, they are already differentiated into the classes Hydrozoa, Anthozoa (comprising already differentiated corals and anemones), Cubozoa (box jellyfish) and Scyphozoa (other jellyfish). Likewise, when molluscs first appear, they are already differentiated into the classes Gastropoda, Bivalvia and Cephalopoda (amongst others), represented today by whelks, scallops and octopuses. The first cephalopods even had jet propulsion and eyes (probably camera eyes). The phylum Brachiopoda – a type of shellfish – already in the Cambrian encompassed eight classes: Lingulata, Phoronata, Craniata, Chileata, Obolellata, Kutorginata, Strophomenata and Rhynchonellata. And so with the other phyla. These radical pre-programmed re-organisations within the basic plan must have taken place invisible to the fossil record during the Precambrian. Evolutionary history after the Cambrian was minor in relation to what went before: the origination of orders and a few further classes within the phyla and of many new species within the already established classes.
Of course, if we abandon the presupposition of interrelatedness and instead suppose that the body plans were present from the beginning, what we are left with is a problem of timing. A long stretch of time preceded the Cambrian, during which bacteria, algae and ‘acritarchs’ (unidentified microscopic plankton but mostly the resting cysts of algae) are well attested. Although they eluded fossilisation, animals also must have existed. Ancestors of the brachiopods, tunicates, onychophora, chelicerates, crustaceans, molluscs, cnidarians, comb jellies, arrow worms, echinoderms, hemichordates, bryozoa, flatworms, roundworms, segmented worms, spiny-headed worms, radiolarians, loricifera and chordates, not to mention the phyla that became extinct, must have existed somewhere and in some form for at least as long as bacteria and algae did.
According to the biblical tradition, the original ocean lay beneath the land (see The antediluvian world). Lakes may have existed at surface level, but since all terrestrial surfaces were destroyed in the Cataclysm, the only aquatic creatures likely to have survived would have been those living at great depth, in the dark. Indeed, a great variety of animals and other organisms still live below the 200 metre-deep zone through which light penetrates.
Following the Cataclysm, seafloor spreading, and thus the generation of ocean crust, was orders of magnitude faster than now, because heat-producing radioactive elements were more abundant and their rate of decay was much higher. Consequently the seas were warmer. Some estimates suggest average temperatures in the Archaean in excess of 60 °C. Submarine volcanism spewed out large volumes of dissolved iron, manganese and sulphur and made all but the uppermost layer of the ocean anoxic and poisonous to animal life. Photosynthesising cyanobacteria made the surface habitable by generating oxygen, which reacted with the iron, manganese and sulphur to precipitate insoluble oxides, which then sank and allowed the oxygen not consumed by the reactions to remain dissolved in the water. Since oxygen solubility decreases with temperature, most of it escaped into the atmosphere. Marine animals will have been confined to the cooler poles.
- the coastal waters were no longer toxic
- oxygen levels at the seafloor were high enough
- the seas had cooled sufficiently
 The earliest animal fossils are a scyphozoan jellyfish (van Iten et al. 2014), a putative medusozoan (Dunn et al. 2022) and an organism tentatively identified as a sponge (Yin et al. 2015), both dating to c. 600 Ma. The record is then blank (though in palaeontology one should never say never) until around 575 Ma, when a strange assortment of soft-bodied multicellular organisms appear known as the Ediacaran Biota. They show up about the same time in many parts of the world, from the Ediacara Hills of Australia to Charnwood Forest in England, initially in deep-water settings. By 555 Ma they had migrated to the shallower shelf (Boag et al. 2018). Some are unattached; some, having a frond-like appearance, are fixed to the seafloor by holdfasts and connected to each other by filaments up to metres long, possibly suggesting that they reproduced by cloning. It is not clear that any were ancestral to later organisms, and most became extinct before the Cambrian. One fossil, the bilaterally symmetrical, leaf-like Dickinsonia, is associated with the remains of cholesterol, reportedly only found in animals. Either Dickinsonia was an animal, or it is evidence that cholesterol does not occur only in animals (which is in fact the case). It had no discernible legs, eyes, mouth or gut, and it does not resemble any Cambrian animal. Arkarua have been an early soft-bodied echinoderm. Kimberella had a gut and fed on algae and bacteria.
The earliest animal fossils are a scyphozoan jellyfish (van Iten et al. 2014), a putative medusozoan (Dunn et al. 2022) and an organism tentatively identified as a sponge (Yin et al. 2015), both dating to c. 600 Ma. The record is then blank (though in palaeontology one should never say never) until around 575 Ma, when a strange assortment of soft-bodied multicellular organisms appear known as the Ediacaran Biota. They show up about the same time in many parts of the world, from the Ediacara Hills of Australia to Charnwood Forest in England, initially in deep-water settings. By 555 Ma they had migrated to the shallower shelf (Boag et al. 2018). Some are unattached; some, having a frond-like appearance, are fixed to the seafloor by holdfasts and connected to each other by filaments up to metres long, possibly suggesting that they reproduced by cloning. It is not clear that any were ancestral to later organisms, and most became extinct before the Cambrian. One fossil, the bilaterally symmetrical, leaf-like Dickinsonia, is associated with the remains of cholesterol, reportedly only found in animals. Either Dickinsonia was an animal, or it is evidence that cholesterol does not occur only in animals (which is in fact the case). It had no discernible legs, eyes, mouth or gut, and it does not resemble any Cambrian animal. Arkarua have been an early soft-bodied echinoderm. Kimberella had a gut and fed on algae and bacteria.
Apart from the sponge, animals with mineralised skeletons and armour first appear around 550 Ma, becoming more abundant in the Cambrian. Their collective name, ‘small shelly fauna,’ reflects the fact that most are only a few millimetres in length and not easily identified. Many seem to be fragments of larger animals such as brachiopods and echinoderms. One of the better known examples, Cloudina, formed extensive reefs consisting of tube-like stacks of nested cones. One function of the shells was to protect against predation. However, borings show that unknown predators had already evolved the ability to overcome these defences: carnivory was a feature of the very earliest examples of animal-inhabited ecosystems, all in carbonate shallow-marine settings.
Meanwhile, though the details remain unclear, there seems to have been an acceleration of plate tectonics. The break-up of the supercontinent called Rodinia was coming to an end, and another amalgamation, chiefly comprising what is now South America and Africa, was nearing its completion. The dispersing continents – North America (including Scotland), Baltica (northern Europe) and Siberia, among others – shifted from various latitudes in the southern hemisphere to latitudes either side of the equator. Avalonia, a microcontinent including England and Wales, migrated during the Cambrian and Ordovician from around 60º S to 40º S. Undersea magmatism was creating the buoyant floors of new oceans, causing sea-levels to rise. The margins of the dispersing continents flooded, to produce vast, shallow, epicontinental seas, far removed from the still hot, toxic, and largely anoxic global ocean. Such isolation enabled shallow waters to reach a new equilibrium with the oxygen-rich atmosphere. Thus the area of habitable marine environments surged. At the same time, zones of upwelling where the deep sea met the shelf dredged up huge quantities of phosphorus, a vital nutrient that is depleted in the upper ocean, and spread them onto the platforms. Owing in part to exceptional conditions of preservation, phosphate deposition peaked in the early Cambrian.
This is the significance of the ‘Great Unconformity’ famously displayed near the bottom of the Grand Canyon, where variably tilted volcanic and sedimentary rocks of Meso- and Neoproterozoic age were planed off as if by a giant saw. Above the unconformity lie horizontal strata of Cambrian age, laid down after a period of extraordinary erosion. Carbon dioxide escaping from the submarine rifts acidified the flood waters, so that the cement between the sedimentary grains dissolved and the rocks disintegrated. Thick blankets of continentally sourced, quartz-rich sandstones and shales were deposited. As a result of the dissolution, huge quantities of calcium entered the ocean, at the same time as elevated rates of seafloor spreading bound up more of its magnesium. In the period 550 to 515 Ma the Mg/Ca ratio of seawater plunged, from 6.1 to 1.2. Alkalinity, in part a function of the Ca and Mg ions in the water, recovered, rising to levels higher than at any other time in the Phanerozoic, allowing thick accumulations of limestone to precipitate, only a small proportion of which consisted of shells. Eventually, by the early Ordovician, most of North America was flooded. Sea-levels globally were high.
The Great Unconformity can be seen in many parts of the world, along with the same sequence of rocks: a basal conglomerate, then quartz-rich sandstones, glauconitic sandstones, shales and finally limestones (Ager 1981). That does not mean that the associated flooding and erosion were synchronous, nor that a time gap exists everywhere below the Cambrian. Indeed, where there is no gap, the point at which one crosses into the Cambrian is often far from clear. After the Cambrian, chronological boundaries are defined by the earliest appearance of organisms (‘index fossils’) that were both wide-ranging and short-lived, on the basis that they spread across the globe from their region of origin more rapidly than radiometric dating has the power to distinguish, and either they rapidly evolved into some other species or they died out. At the beginning of the Cambrian, however, fossils are rare and even higher up there are no widespread, rapidly evolving organisms to provide a basis for correlation between continents (Zhang et al. 2017). The Cambrian Explosion does not really get underway until about a fifth of the way into the period, around 530 Ma and lasted more like 20 Ma than Eldredge’s ‘ten million’. In the Precambrian, where fossils are even scarcer, time boundaries are simply round numbers on the chronometric scale.
Despite the scarcity of fossils, the boundary between the Precambrian and the Cambrian is defined by the first appearance of the distinctive burrow Treptichnus pedum, probably made by a priapulid worm. Whether this marks a true time-line remains to be demonstrated. Most of the places where T. pedum appears, however, lack volcanic rocks suitable for dating and, in the two places where there are such rocks, the dates do not agree. In Oman the boundary is dated to 541 Ma, in Namibia to 538.7 Ma, more than 2 Ma later. Priapulids are one of the few phyla that do not (now) have planktonic or free-swimming larvae, so if they originated from a single region just before the Cambrian, they may have taken longer to disperse than other types of animal. On the other hand, we should allow for the possibility that their ancestors – living in some unknown manner above the seafloor – were globally distributed before they showed up in the fossil record.
The dating problem at this time is in fact systemic. The lower and middle part of the Cambrian is the only interval in the Phanerozoic where it has proved impossible to correlate strata on different continents on the basis of fossils. When trilobites first appear around the world, they already differ from each other – not just at the species level, even at the family level. That is true across the board. Chengjiang and Qingjiang are two contemporaneous (518 Ma) and exceptionally fossiliferous sites in China just 1000 km apart, yet Qingjiang shares less than 10% of its species in common with Chengjiang – partly perhaps because Chengjiang’s location on the shelf was closer to shore, but partly because of endemism. Although the larvae of many phyla are capable of riding on currents across entire oceans, there had not been time enough for fauna to mix. The assumption that the occurrence of the same species of trilobite or sponge marks the same interval everywhere does not hold (and these organisms probably did have free-swimming larvae).
Evidently the animals which brought about the Cambrian revolution did not come into being ex nihilo. Nor is it plausible that some of the Ediacaran organisms might have been their ancestors. The interval between those organisms’ first appearance and that of the Cambrian phyla was far too short for slight, successive modifications to have brought about the necessary across-the-board transformations – one might as well believe in natural magic. More viable explanations for the apparent absence of ancestors are: (1) the animals were too small and too few in number to have made a mark on the fossil record, (2) they lacked fossilisable hard parts, such as bones, or shells, and (3) they were mobile rather than sessile, swimming at the surface far away from potential burial. Some change in global conditions must have triggered, in nearly every phylum, a fundamental change in behaviour and physiology.
Skeletal hard parts are found in the majority of animal phyla, some consisting of aragonite (a form of calcium carbonate), some of calcite (another form of calcium carbonate), some of calcium phosphate, some of silica. Strange though the idea may appear at first, their absence from the fossil record before 550 Ma suggests that animals acquired hard parts in the course of evolution. Palaeontologists refer to this change as ‘biomineralisation’.
Undoubtedly the most striking instance of delayed biomineralisation is the late appearance of modern corals, order Scleractinia, within the class Anthozoa. The two large orders of corals that, in the Ordovician, had already acquired hard skeletons became extinct at the end of the Permian. Scleractinians did not hit the fossil record until the following period, in the mid Triassic, by which time they already comprised three suborders and nine or ten families. The majority view is that they originated as soft-bodied forms in the Ordovician, about the same time as the other two orders. Since ocean carbonate saturation decreases with depth, possibly they were restricted to the deep sea during the Palaeozoic and began to migrate to shallower levels only in the Mesozoic.
If phyla are all related to each other and evolution is a chance process, the presumption should be that the genetic instructions for making carbonate skeletons evolved just once, after which the biomineralising group would have differentiated from the non-biomineralising group along a separate path. The presumption can be tested, because phyla are arranged in a single evolutionary tree on the basis of how similar they are in their DNA and in how they develop as embryos, factors believed to probe relationships at a much more fundamental level than whether they biomineralise. What one finds is that the biomineralisers are scattered irregularly through the tree (Murdock & Donoghue 2014). Biomineralisers and non-biomineralisers occur even within the same phylum: within comb jellies, for instance (Ou et al. 2015). Thus the genetic instructions for making hard parts must have arisen independently – at least 14 times. Indeed, phosphatic skeletons alone are thought to have evolved more than 10 times, and all during the early Cambrian (Bengtson & Conway Morris 1992). Which, then, is the more likely scenario, that the same mechanism evolved multiple times and more or less simultaneously by chance, or that the different lineages were each created with those instructions? Since the former scenario ascribes to chance just the same limitless inventiveness as a supernatural Creator, not to mention the same invisibility, it is in reality no more than a contradiction in terms. If the phenomenon requiring explanation cannot reasonably be attributed to chance, only the supernatural option remains.
Although there are degrees of softness, in general soft parts get fossilised (to some degree) only where preservational conditions are exceptional. Examples of soft-bodied animals fossilised in the Cambrian include a 0.5 mm anemone, various worm taxa, jellyfish and a cephalopod. In most cases, however, we only become aware of a class or phylum at the point when it began to biomineralise. One moment the group was invisible to the fossil record, the next, the extent to which it had biomineralised was already at a maximum. The genetic instructions to form a skeleton were switched on as a unit and affected the whole organism. From the moment brachiopods came on the scene, their two valves were fully formed and interlocked perfectly. From the moment trilobites appeared, every part of the skeleton was mineralised, as were the calcite lenses of their eyes. In response to hormonal signals they already had the ability to moult, sloughing off their old carapaces along predetermined suture lines, and in their place to grow bigger ones, together with additional thoracic segments.
 Despite their morphological differences, one surprising thing that nearly all marine invertebrate phyla have in common, above a certain size, is that they start off as larvae: tiny, usually soft-bodied swimmers. Only after metamorphosing into an entirely different form do they restrict themselves to life on the seafloor. If ‘basic body plans’ are not predicted in Darwinian evolution, still less is it predicted that nearly all the phyla have at least two body plans, the larva being just as distinct as the adult. Sponges, worms (encompassing several phyla), molluscs, cnidarians (e.g. corals), echinoderms, brachiopods and loricifera (microscopic sediment-dwellers resembling stalk-filled vases) all have a larval stage, and the larvae are extremely diverse. As one report puts it, ‘the diversity of larval forms is so overwhelming that it almost defies biologists with questions’ (Hejnol & Vellutini 2017). Nemertean worm larvae are ready to metamorphose after 5-8 weeks, whereupon the juvenile develops inside the larva from a series of isolated rudiments and consumes the remaining body. Some larvae go through more than one stage: barnacles, for example, go through two. The first, called the nauplius, has a thin calcareous shell, a single eye and goes through five moults – itself a remarkable phenomenon – before transforming into the cypris, whose role is to look for a suitable substrate (Young 1999). ‘Suitable’ might be sediment of a particular grain size or texture, or one already occupied by individuals of the same species or (in the case of predators) of different species. To avoid predation, many larvae rise towards the surface at night and seek deeper water during the day. As they grow, they become less responsive to light and gravitate to the floor.
Despite their morphological differences, one surprising thing that nearly all marine invertebrate phyla have in common, above a certain size, is that they start off as larvae: tiny, usually soft-bodied swimmers. Only after metamorphosing into an entirely different form do they restrict themselves to life on the seafloor. If ‘basic body plans’ are not predicted in Darwinian evolution, still less is it predicted that nearly all the phyla have at least two body plans, the larva being just as distinct as the adult. Sponges, worms (encompassing several phyla), molluscs, cnidarians (e.g. corals), echinoderms, brachiopods and loricifera (microscopic sediment-dwellers resembling stalk-filled vases) all have a larval stage, and the larvae are extremely diverse. As one report puts it, ‘the diversity of larval forms is so overwhelming that it almost defies biologists with questions’ (Hejnol & Vellutini 2017). Nemertean worm larvae are ready to metamorphose after 5-8 weeks, whereupon the juvenile develops inside the larva from a series of isolated rudiments and consumes the remaining body. Some larvae go through more than one stage: barnacles, for example, go through two. The first, called the nauplius, has a thin calcareous shell, a single eye and goes through five moults – itself a remarkable phenomenon – before transforming into the cypris, whose role is to look for a suitable substrate (Young 1999). ‘Suitable’ might be sediment of a particular grain size or texture, or one already occupied by individuals of the same species or (in the case of predators) of different species. To avoid predation, many larvae rise towards the surface at night and seek deeper water during the day. As they grow, they become less responsive to light and gravitate to the floor.
Metamorphosis occurs in such cases when neurosensory cells detect a specific environmental cue and stimulate the release of chemicals into the nervous system (Ueda et al. 2016). These then activate a cascade of signals which induce the larva to settle and begin the transformation into an adult. Signalling systems differ from one family to another, but in most of them internally synthesised nitric oxide plays a key role. Metamorphosis is repressed when the neurosensory cells maintain high levels of the gas and promoted when synthesis of nitric oxide drops.
Metamorphosis is pre-programmed transformation of the individual. To suppose that such programming could have arisen from ‘numerous, successive, slight modifications’ (Darwin’s phrase), each occurring by chance, is to fly in the face of the message that is actually presented. How could the hypothetical modifications each have constituted an advantage in relation to a transformation that was all or nothing? Anything along the way would not have been viable. The transition is kept as brief as possible precisely because the organism during that time is not viable. Even if a long series of evolutionary steps had given rise to the adult form, how could the transition subsequently ever have been telescoped into a few hours? Species evolution and the development of an individual are separate things. By definition, natural selection leaves the previous steps behind, because they are less ‘fit’. In species that metamorphose, the larval stage is not left behind though it can happen (e.g. with some echinoderms and sea squirts) that some species lose the larval stage.
Larvae enable sessile and burrowing animals to colonise areas beyond their immediate habitat. The ability to colonise long-range is of course of no little significance in relation to the Cambrian and Ordovician, when fauna (it is postulated) migrated from high to low latitudes and (it is well established) quickly became more cosmopolitan. The non-existence of worm burrows earlier than 560 Ma clearly implies that the ancestors of the worm phyla lived as larvae above the sediment. Animals did not get fossilised in the Precambrian, we may surmise, because the environment was giving signals that inhibited them from settling and metamorphosing, for example because oxygen concentrations were too low. Unlike their modern equivalents, they must have been able to reproduce. In this respect a modern analogue might be certain species of salamander, which become sexually mature as larvae and do not metamorphose. The fact that some lineages have re-evolved metamorphosis after previously bypassing the larval stage (Chippendale et al. 2004) supports the inference that this ability was programmed into organisms from the beginning and could be switched on or off.
Very occasionally, notably at Qingjiang, unclassifiable ‘submillimeter- to millimetre-sized, delicate, larval or juvenile forms’ did get to be fossilised. As with most instances of soft-body preservation, they owed their preservation to episodic turbidity flows transporting them downslope to levels where the lack of oxygen inhibited decomposition. In addition, we know of a few non-mobile taxa from the latest Precambrian that were soft-bodied in clastic environments but skeletonised in carbonate environments (Wood et al. 2017). The morphology of each pair was almost the same. In most cases, however, metamorphosis and the development of hard parts probably went hand in hand. Some larvae have been observed to grow calcite skeletons prior to metamorphosis, giving them ballast, so that they sink through the water column. Similarly the larvae of trilobites, fossils of which go back as far as the adults, had calcareous shells.
The main environmental cue for the transformation would have been higher levels of oxygen in the water, since the production of collagen and skeletal microstructures required more energy. So did the larger body size and complex musculature of the biomineralised adult form. Carbonate rock sections that contain chemical evidence of fluctuating oxygen levels indicate that small skeletal organisms preferred waters that were well-oxygenated. Large skeletal organisms lived only in such conditions (Tostevin et al. 2016).
Another cue would have been a change in ocean chemistry. As we have noted, calcium concentrations rose dramatically through the early and middle Cambrian. Naturally it is easier to secrete calcium carbonate when calcium concentrations in the seawater are high. During times of high Mg/Ca (higher than 2), the mineral takes the form of aragonite; during times of low Mg/Ca, it precipitates as calcite. The threshold was crossed around 525 Ma. Before then, animal classes that secreted shells made them out of aragonite or, less commonly, calcium phosphate. The majority waited until after 525 Ma, when they made them out of calcite. In no case did a taxon start by making them out of aragonite and switch to calcite when Mg/Ca fell. Those that secreted calcite did not biomineralise earlier because they were not programmed to do so.
 Burrowers had already begun to leave traces before the boundary, mostly in the form of horizontal meanderings, less commonly as vertical and U-shaped burrows. Small body fossils such as the stumpy Ikaria, 1-1.5 mm wide (smaller than a grain of rice), and the elongate Yilingia, up to 2.6 cm wide, have also been discovered from below the boundary. They were ecological pioneers. Faecal pellets dropped by zooplankton in the water column attracted animals that grazed and burrowed, and as they consumed the droppings, they churned up the sediment, aerated it and fertilised it. This, in turn, enabled other burrowers to live in and feed off still greater depths of sediment. Conversely, oxygenation helped buried organic matter to decompose and buried phosphate to be recycled. Low rates of deposition were another prerequisite. The chances of survival were slim if the seafloor was no sooner colonised than buried. In such ways barren seafloors were turned into habitable environments for almost the full range of seafloor-dwelling organisms.
Burrowers had already begun to leave traces before the boundary, mostly in the form of horizontal meanderings, less commonly as vertical and U-shaped burrows. Small body fossils such as the stumpy Ikaria, 1-1.5 mm wide (smaller than a grain of rice), and the elongate Yilingia, up to 2.6 cm wide, have also been discovered from below the boundary. They were ecological pioneers. Faecal pellets dropped by zooplankton in the water column attracted animals that grazed and burrowed, and as they consumed the droppings, they churned up the sediment, aerated it and fertilised it. This, in turn, enabled other burrowers to live in and feed off still greater depths of sediment. Conversely, oxygenation helped buried organic matter to decompose and buried phosphate to be recycled. Low rates of deposition were another prerequisite. The chances of survival were slim if the seafloor was no sooner colonised than buried. In such ways barren seafloors were turned into habitable environments for almost the full range of seafloor-dwelling organisms.
Marine organisms appeared successively – first phytoplankton (the photosynthesising primary producers), then zooplankton, then seafloor-dwelling herbivores and immobile filter-feeders, then swimming and seafloor-dwelling carnivores and deposit-feeders, finally large predators. Sessile organisms such as sponges and corals themselves modified their environments by building reefs, creating ecological niches for other organisms, and at all levels of the ecological pyramid species multiplied at the same time as the levels themselves multiplied. Others, such as sea-lilies and bryozoans, encrusted the cemented floors called hardgrounds, before sedimentation resumed and smothered the animals and prevented further build-up. The explosive appearance of marine life was thus a staggered rather than instant phenomenon. In the Cambrian marine animal life was mostly restricted to habitats in and immediately above the seafloor; by the Ordovician almost the entire water column was filled with organisms. This is the true meaning of the order of fossils. Organisms higher up the food chain depended on those lower down and were not programmed to reproduce as numerously. In all its diversity marine life was designed as a complex community.
 Food webs in the Cambrian were ‘remarkably similar’ in structure to modern food webs (Dunne et al. 2008). Fundamentally, marine food chains changed little over time, just as, fundamentally, the organisms that composed them changed little. The disparate organisms that appeared in the Cambrian were linked by food chains, not evolutionary chains. There is no evidence that zooplankton evolved from bacteria, or that worms, molluscs, sponges and so on evolved from zooplankton.
Food webs in the Cambrian were ‘remarkably similar’ in structure to modern food webs (Dunne et al. 2008). Fundamentally, marine food chains changed little over time, just as, fundamentally, the organisms that composed them changed little. The disparate organisms that appeared in the Cambrian were linked by food chains, not evolutionary chains. There is no evidence that zooplankton evolved from bacteria, or that worms, molluscs, sponges and so on evolved from zooplankton.

Darwin’s theory of evolution requires the evidence of ‘numerous, fine, intermediate, fossil links’. He imagined that in the vast ages before the Cambrian the world must have ’swarmed’ with living creatures. But what we find is revolution, not evolution: an explosion of life forms as continental margins flooded and began to be colonised by phyla that must have pre-existed, invisibly, as tiny soft-bodied organisms in the open sea – immigrants, one surmises, from the polar regions. Starting with bacteria and climaxing with sharks, it was an ecological progression, something that occurred over thousands of years, not three thousand million. The phyla, present from the beginning, became visible as large areas of continent flooded and species responded to a variety of factors: an increase in nutrients released by continental erosion, an increase in dissolved calcium, an increase in oxygen levels. The response was to metamorphose from tiny larvae to much larger adults, to biomineralise, to take up a settled existence on the seafloor or prey on others that had done so. It was only when thrown into close proximity with each other that organisms began to compete. Environments multiplied, dividing into ever finer ecological niches, and as they multiplied, so did the species that filled them. Deeper levels of the sea became inhabited as they became more oxygenated.







